This warning saved my life
Vaquedoso
- 0 Posts
- 22 Comments
 152·2 months ago
152·2 months agoI really liked season 1, so I’m looking forward to the second
 69·4 months ago
69·4 months ago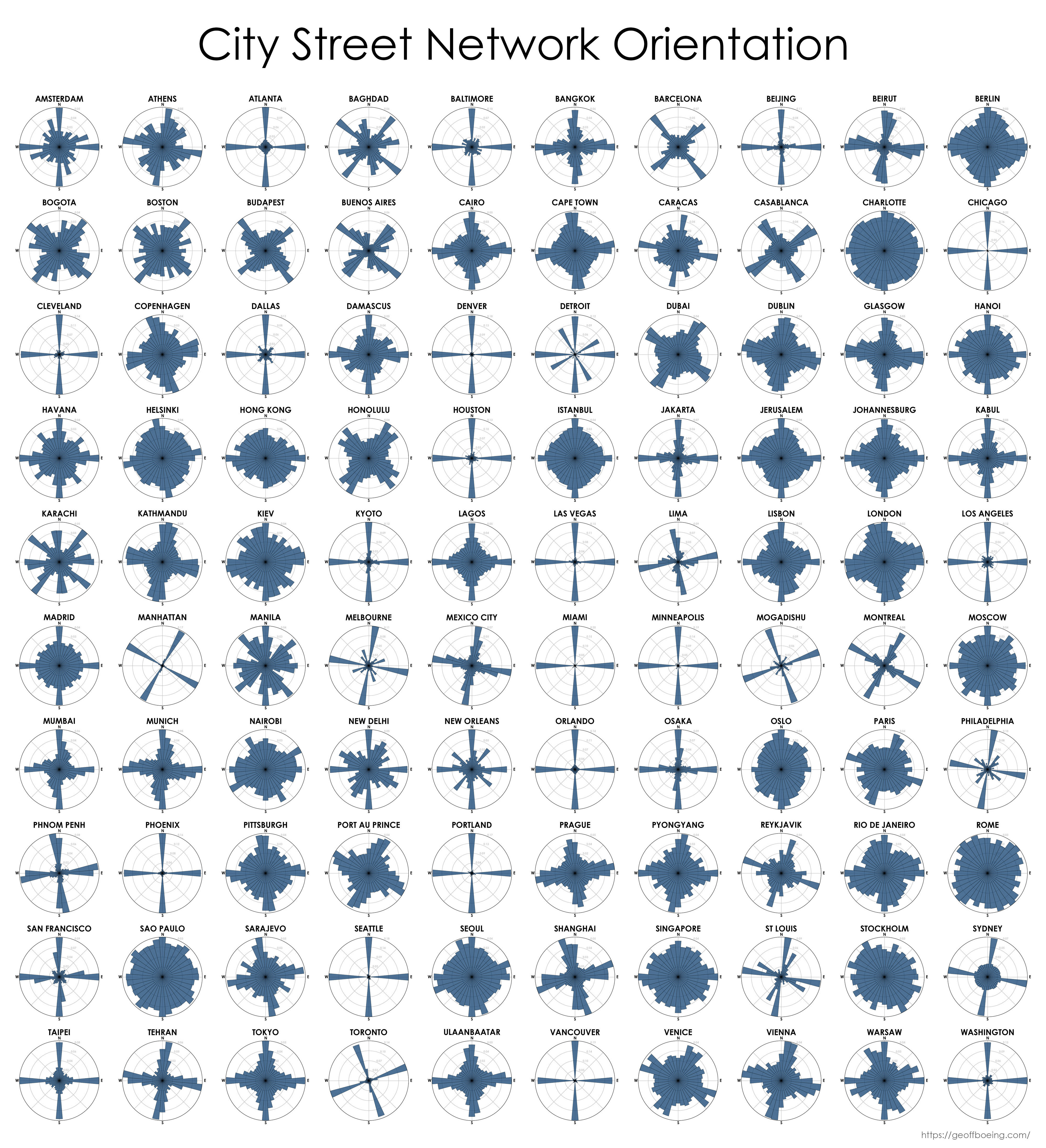
Here’s one that includes more cities around the world
 104·4 months ago
104·4 months agoI’m not here to argue with you, rather than to point out it’s disingenious to say it’s not hard work. Also, there is actually an incentive to get lower prices, for if they weren’t trying to get one people would choose another realtor. Also, you don’t have to censor the world realtor.

 19·5 months ago
19·5 months agoThey might have not suffered the noise of cars as much as today, but accordig to that photo they did constantly suffer the noise of lowercase a everywhere
There’s really no debate about it.
We discarded Descartes
Think of a ball pit filled with balls of different colours. After a session of intense playing in it, presumably all the balls will have been mixed up. It’s now in a state of high entropy, where all of the balls are evenly distributed across the pit. But now, what if I wanted to enter the pit and sort the balls by colour? We would then have a corner of blue balls, a corner of red ball, etc. we have invested energy in the system and sorted the balls by colour, they are now ‘ordered’, and in a low entropy state. We know this state won’t last long though, once we get it and play the balls are gonna end up inevitably mixed up once again, the balls are going to end up in a high entropy equilibrium again.
It’s not a ‘scary cosmic horror’, it’s not even a force. It’s a principle, a consequence of the second law of thermodynamics. In any isolated system, its energy will always strive towards balance, (eg, in a isolated room where one corner is hot and another cold, the temperature eventually will balance in a equilibrium). From this process of homogenization is where we derive the term entropy. Any homogeneous system has high entropy, for instance, as it is the tendency in any system for its components to be uniform. As a descriptor, one can measure in any system its level of entropy.
In regards to your question, entropy doesn’t have a will of its own, so it wouldn’t have set out to stop the formation of the solar system. But eventually, the solar system WILL reach a state of high entropy, once the sun (the one providing the energy that keeps the solar system going) runs out of fuel and goes supernovae
 5·7 months ago
5·7 months agoThese are photographs, actually
deleted by creator
Is that Sam Reich’s dad?

 8·10 months ago
8·10 months agoThat’s what I’m saying, it’s incredible! Here where I live fast food places don’t offer free refills, you have to buy another soda if you want more

 341·10 months ago
341·10 months agoIt always amazes me that in the USA you can go and get a free refill at McDonald’s. Like you can get unlimited soda just by buying a cup?

 32·11 months ago
32·11 months agoYo por ti perdí la calma y casi pierdo hasta la cama 🎶

 91·11 months ago
91·11 months agoThis article is behind a paywall

 2·11 months ago
2·11 months agoI don’t mean to start a discussion here, but this is your interpretation and it’s valid. But galadriel’s character has contradictory history depending on your sources (even regarding the kinslaying). And it’s debated even between Tolkien’s scholars the extend at which she can ‘sense evil’. After all, she herself was deceived by saruman after his corruption during the third age.
In your last paragraph you say ‘nothing about her character in the series has any respect for Tolkien’s work’. That’s simply hyperbole, and arguably not true, as even a surface level reading can prove otherwise. Such words are not Tolkien’s way.
Anyway, I don’t want you to change your mind, just want you to be aware of the possibility of other interpretations. Take care!

 3·11 months ago
3·11 months agoDo let me know what you think of it!

 61·11 months ago
61·11 months agoHonestly I don’t really care, I’m more inclined to strategy and 4x games.
If you don mind me recommending a game, check out against the storm, it’s a city builder with rogue like elements, and it came out recently out of early access, it’s reaaaaally addicting



I also say sun in place of sun